Oxygen activation by copper camphor complexes†
Abstract
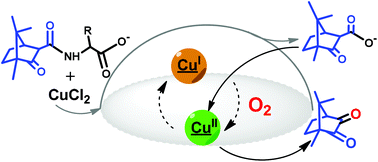
Cleavage of the carboximide CN bond promoted by reaction of camphor carboximide hydrochlorides (OC10H15CONHCH2COOLi(H2O)2·2HCl (2) or OC10H15CONHCH(CH2Ph)COOLi(H2O)·HCl (3)) with CuCl2 leads to the corresponding amino acid complexes ([Cu(H2NCH2COO)2] (I) and [Cu{H2NCH(CH2Ph)COO}2]·(H2O) (II)), and the camphor carboxylate residue (OC10H15COO−) which is catalytically oxidized to camphorquinone by oxygen from air. The process is mediated by coordination of the camphor carboxylate to copper. The reaction of the camphor carboximide hydrochloride (OC10H15CONH(CH2)2COOLi(H2O)·HCl) (4) with CuCl2 follows a different trend. In this case the CN camphor carboximide bond remains intact and the complexes [CuCl{OC10H15CONH(CH2)2COOLi(H2O)}] (III) and [{CuCl2}2{OC10H15CONH(CH2)2COOLi(H2O)}] (IV) form. However, a redox process also occurs, since formation of III requires Cu(II) → Cu(I) reduction as confirmed by X-ray photoelectron spectroscopy and cyclic voltammetry. A related reduction process was identified in the formation of [CuCl(OC10H15COOLi)] (V) from CuCl2 upon reaction with lithium camphor carboxylate (OC10H15COOLi) under nitrogen. The above results show that electron transfer is highly facilitated in the copper camphor carboximide/carboxylate system. Such an ability was used to activate oxygen from air and promote the oxidation of ethylacetoacetate to pyruvate using V as the catalyst.
- This article is part of the themed collection: HOT articles in Inorganic Chemistry Frontiers for 2015

 Please wait while we load your content...
Please wait while we load your content...