New tricopper(ii) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes†
Abstract
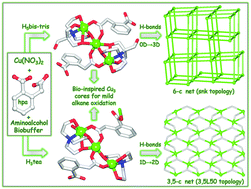
Two new crystalline materials [Cu3(μ2-H3bis-tris)2(μ2-Hhpa)2]·H2O (1) and [Cu3(μ2-H2tea)2(μ2-hpa)(μ3-hpa)]n (2) bearing distinct tricopper(II) cores were easily generated by the aqueous medium self-assembly method from copper(II) nitrate, bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane (H5bis-tris) or triethanolamine (H3tea) aminoalcohol biobuffers and homophthalic acid (H2hpa). The obtained products were characterised by IR, UV-vis and EPR spectroscopy, ESI-MS(±), thermogravimetric, elemental and single crystal X-ray diffraction analysis. Apart from possessing different dimensionality, the crystal structures of the discrete 0D trimer 1 and the zigzag 1D coordination polymer 2 show distinct symmetric [Cu3(μ-O)4(μ-COO)2] and asymmetric [Cu3(μ-O)3(μ-COO)2] tricopper(II) cores, respectively. An intense pattern of intermolecular O–H⋯O hydrogen bonds provides a 0D → 3D (1) or 1D → 2D (2) extension of the structures into intricate topologically unique H-bonded nets. After additional simplification, these were classified as a uninodal 6-connected 3D framework with the snk topology in 1 and a binodal 3,5-connected 2D layer with the 3,5L50 topology in 2. Variable-temperature magnetic susceptibility studies indicate a predominant ferromagnetic coupling [J = 39.1(1) and 29.5(1) cm−1 for 1 and 2, respectively] within the mixed-bridged tricopper(II) cores. Both compounds 1 and 2 were also applied as rather efficient bio-inspired pre-catalysts for the mild homogeneous oxidation, by aqueous H2O2 at 50 °C in acidic MeCN–H2O medium, of cyclic (cyclopentane, cyclohexane, cycloheptane and cyclooctane) and linear (n-pentane, n-hexane, n-heptane and n-octane) alkanes to the corresponding alcohols and ketones with overall yields up to 26% based on the alkane. The effects of different reaction parameters (type of pre-catalyst and acid promoter, reaction time and substrate scope) and various selectivity features were investigated and discussed, supporting a free-radical mechanism in the present alkane oxidations.
- This article is part of the themed collection: Crystal engineering for molecular materials

 Please wait while we load your content...
Please wait while we load your content...