Role of 4,4′-bipyridine versus longer spacers 4,4′-azobipyridine, 1,2-bis(4-pyridyl)ethylene, and 1,2-bis(pyridin-3-ylmethylene)hydrazine in the formation of thermally labile metallophosphate coordination polymers†
Abstract
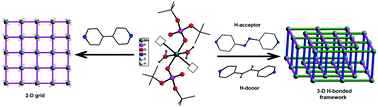
One dimensional metallophosphate coordination polymers {[M(dtbp)2(azopy)(H2O)2]·(azopy)}x (M = Mn (1); Co (2); Cu (3); Cd (4)) have been synthesized from the reaction of a suitable metal precursor with di-tert-butylphosphate (dtbp-H) in the presence of ditopic linker 4,4′-azobipyridine (azopy) in a 1 : 2 : 2 stoichiometric ratio. Isostructural compounds 1–4 have been characterized by analytical and spectroscopic methods and single crystal X-ray diffraction studies. Single crystal X-ray diffraction measurements further reveal that compounds 1–3 (all C2/c) and 4 (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) are linear 1D coordination polymers. The uncoordinated 4,4′-azobipyridine in the lattice is responsible for the conversion of these 1D coordination polymers into 3D supramolecular assemblies through O–H⋯N hydrogen bonding interactions between coordinated water and N-centers of an azopy ligand. Similar reactions carried out using 1,2-bis(4-pyridyl)ethylene (bpe) as the linker yielded compounds having the formula {[M(dtbp)2(bpe)(H2O)2]·(bpe)}x (M = Mn (5); Co (6); Cu (7); Ni (8)). Compounds 5–8 have been characterized by analytical and spectroscopic methods. Preliminary single crystal X-ray diffraction studies carried out on poorly diffracting crystals of 5 and 6 establish their isostructural nature to 1–4, also displaying a similar supramolecular aggregation behaviour. A longer ditopic N,N′-donor ligand, 1,2-bis(pyridin-3-ylmethylene)hydrazine (bph), has been used in place of bpe/azoby to synthesize 1-dimensional coordination polymers [{M(bph)(H2O)4}{(dtbp)2}] (M = Ni (9) and M = Co (10)) and [Cd(bph)3(dtbp)2]n (11) which have completely different structural motifs when compared to 1–4.
) are linear 1D coordination polymers. The uncoordinated 4,4′-azobipyridine in the lattice is responsible for the conversion of these 1D coordination polymers into 3D supramolecular assemblies through O–H⋯N hydrogen bonding interactions between coordinated water and N-centers of an azopy ligand. Similar reactions carried out using 1,2-bis(4-pyridyl)ethylene (bpe) as the linker yielded compounds having the formula {[M(dtbp)2(bpe)(H2O)2]·(bpe)}x (M = Mn (5); Co (6); Cu (7); Ni (8)). Compounds 5–8 have been characterized by analytical and spectroscopic methods. Preliminary single crystal X-ray diffraction studies carried out on poorly diffracting crystals of 5 and 6 establish their isostructural nature to 1–4, also displaying a similar supramolecular aggregation behaviour. A longer ditopic N,N′-donor ligand, 1,2-bis(pyridin-3-ylmethylene)hydrazine (bph), has been used in place of bpe/azoby to synthesize 1-dimensional coordination polymers [{M(bph)(H2O)4}{(dtbp)2}] (M = Ni (9) and M = Co (10)) and [Cd(bph)3(dtbp)2]n (11) which have completely different structural motifs when compared to 1–4.


 Please wait while we load your content...
Please wait while we load your content...