Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers
Abstract
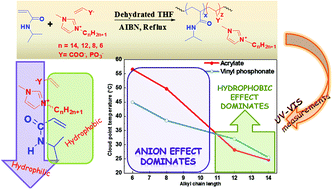
Poly(N-isopropylacrylamide), PNIPAM, is a thermoresponsive polymer widely known for its lower critical solution temperature (LCST) phenomenon at 32 °C in aqueous solutions. Precise tuning of the LCST of PNIPAM to a broader temperature range offers a larger window of applications especially in the field of biotechnology and nanotechnology. A series of free radical random copolymerizations between N-isopropylacrylamide (NIPAM) and various imidazolium based ionic liquids (ILs) were conducted. IL structures were varied in terms of their alkyl chain length at the N-3 (or N-1) position of the imidazolium ring and counter anion. The LCST behavior of the aqueous solutions of the copolymers was investigated through UV-VIS transmission measurements. The results confirm that introduction of IL into the PNIPAM offers a wide range of LCST behaviors with a synergism between the hydrophobic part of the ionic liquid and the basic strength of the counter anion, probably by varying the hydrogen bonding abilities of the copolymer.

 Please wait while we load your content...
Please wait while we load your content...