Ion pairing effects in the zwitterionic ring opening polymerization of δ-valerolactone†
Abstract
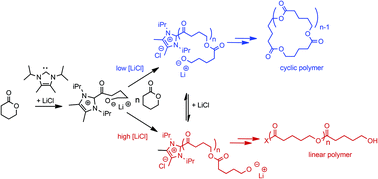
The ring-opening polymerization (ZROP) of δ-valerolactone (VL) by N-heterocyclic carbenes (NHC) in the absence of alcohol initiators generates cyclic poly(valerolactones). A zwitterionic mechanism has been proposed where the propagating alkoxide anion undergoes ion-pairing with the acyl-imidazolium polymer chain end. To evaluate the consequences of zwitterionic ion-pairing, the influence of added LiCl to the NHC-mediated ZROP of δ-valerolactone (VL) in THF was investigated. In THF in the absence of LiCl, the molecular weights of the resultant p(VL) are higher than that predicted from the initial ratio of [VL]0/[NHC]0 and the molecular weight distributions are bimodal and broad (Mw/Mn = 1.35). In the presence of 1.0 M LiCl, the molecular weights of the p(VL) match that predicted from [VL]0/[NHC]0 and the molecular weight distributions are monomodal and narrow (Mw/Mn = 1.08–1.26). The presence of LiCl also influences both the rate of polymerization and the topology of the resultant poly(valerolactones). In the absence of LiCl, cyclic poly(valerolactones) are isolated upon aqueous methanol work-up, but at high LiCl concentrations, only linear chains are produced. These results imply that zwitterionic ion-pairs can be readily broken up by added LiCl.

 Please wait while we load your content...
Please wait while we load your content...