Poly(lactide)-block-poly(ε-caprolactone-co-ε-decalactone)-block-poly(lactide) copolymer elastomers†
Abstract
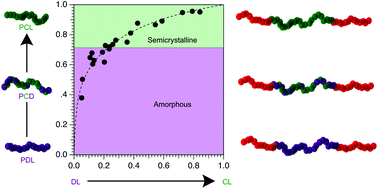
Batch ring opening transesterification copolymerization of ε-caprolactone and ε-decalactone was used to generate statistical copolymers over a wide range of compositions and molar masses. Reactivity ratios determined for this monomer pair, rCL = 5.9 and rDL = 0.03, reveal ε-caprolactone is added preferentially regardless of the propagating chain end. Relative to poly(ε-caprolactone) the crystallinity and melting point of these statistical copolymers were depressed by the addition of ε-decalactone; copolymers containing greater than 31 mol% (46 wt%) ε-decalactone were amorphous. Poly(lactide)-block-poly(ε-caprolactone-co-ε-decalactone)-block-poly(lactide) triblock polymers were also prepared and used to explore the influence of midblock composition on the temperature dependent Flory-Huggins interaction parameter (χ). In addition, uniaxial extension tests were used to determine the effects of midblock composition, poly(lactide) content, and molar mass on the mechanical properties of these new elastomeric triblocks.

 Please wait while we load your content...
Please wait while we load your content...