Facile synthesis of cyclosiloxane-based polymers for hybrid film formation†
Abstract
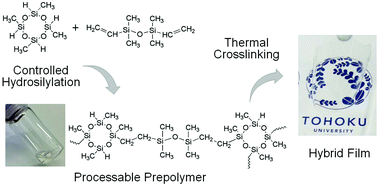
A straightforward synthesis of cyclosiloxane-based hybrid polymers and preparation of hybrid network polymer films were demonstrated. Cyclosiloxane-based hybrid polymers were prepared from one-step hydrosilylation of tetrasilane-functional cyclosiloxanes with divinyl-terminated dimethylsiloxane monomers. Colorless and soluble hybrid polymers were obtained by controlling synthesis parameters such as the initial monomer ratio and the total monomer concentration. The 1H, 29Si NMR and FT–IR results revealed that the resulting hybrid polymers took practically linear structures with residual silane groups that enable us to crosslink hybrid polymers through subsequent condensation reactions with multi-step heat treatments. Consequently, flexible, thermally mechanically robust, optically transparent free-standing hybrid network polymer films were obtained with more than 90% optical transparency (0.2 mm thickness) in the UV–visible light wavelength region (>220 nm). Moreover, they had good thermal stability (approximately 300 °C) and approximately 400 MPa Young's modulus. Self-crosslinking reaction of residual Si–H groups in hybrid polymers contributed to the enhanced thermal and mechanical properties of hybrid polymer films because of the siloxane linkage conversion from R2SiO to the RSiO3/2 framework.

 Please wait while we load your content...
Please wait while we load your content...