Synthetic strategy for preparing chiral double-semicrystalline polyether block copolymers†
Abstract
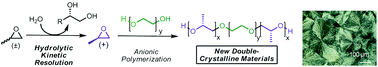
We report an effective strategy for the synthesis of semi-crystalline block copolyethers with well-defined architecture and stereochemistry. As an exemplary system, triblock copolymers containing either atactic (racemic) or isotactic (R or S) poly(propylene oxide) end blocks with a central poly(ethylene oxide) mid-block were prepared by anionic ring-opening procedures. Stereochemical control was achieved by an initial hydrolytic kinetic resolution of racemic terminal epoxides followed by anionic ring-opening polymerization of the enantiopure monomer feedstock. The resultant triblock copolymers were highly isotactic (meso triads [mm]% ∼ 90%) with optical microscopy, differential scanning calorimetry, wide angle X-ray scattering and small angle X-ray scattering being used to probe the impact of the isotacticity on the resultant polymer and hydrogel properties.

 Please wait while we load your content...
Please wait while we load your content...