Semibranched polyglycidols as “fillers” in polycarbonate hydrogels to tune hydrophobic drug release
Abstract
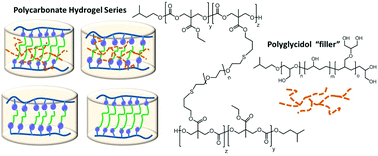
We report on the synthesis of polycarbonate based hydrogels that contain semibranched polyglycidols entrapped into the polycarbonate-diethylene oxide matrix. The primary OH groups of the polyglycidol can also react in a transesterification reaction to form reconfigurable crosslinked materials. We first synthesized allyl and ethylene-oxide functionalized linear polycarbonates with Sn(OTf)2 as catalyst and isoamyl alcohol as initiator. In a reaction with dithiol ethylene oxide and dithiol (poly ethylene oxide) 1.5k, a crosslinked network was formed via thiolene click reactions in the presence or absence of semibranched polyglycidols. The resulting four hydrogels were analyzed for their swelling capabilities, mechanical properties and degradation in phosphate buffered saline. Paclitaxel was chosen as a model drug to study the drug release from these two carriers and was incorporated during the crosslinking reaction. The presence of the polyglycidol as well as the length of the dithiol crosslinker influenced the swelling capabilities, were responsible for a varied drug release behavior and the remarkable stress resistance. The present work introduces and compares polyglycidols as components in polycarbonate crosslinked materials that are either entrapped or covalently attached to the polycarbonate backbone to establish models of structure property relationships for hydrogels used for controlled drug delivery in vivo.

 Please wait while we load your content...
Please wait while we load your content...