Photo-Fries rearrangement of aryl acetamides: regioselectivity induced by the aqueous micellar green environment†
Abstract
Photochemical reactions tend to give more than one photoproduct. However, such a reaction can be a powerful synthetic tool when it is possible to conduct it in regioselective conditions yielding a single photoproduct. Water–surfactant solutions as reaction media can be considered as an approach in this context because they show products with different features than those from isotropic solutions. Here we describe results obtained from studying the effect on the prototypical photoreaction, known as the photo-Fries reaction of several substituted acetanilides and α-naphthyl acetamide within surfactant micelles (ionic and non-ionic micelles). This reaction involves homolytic cleavage of a C–N bond to yield a singlet radical pair. The surfactant micelles control the rotational and translational mobility of the radical pair, resulting in noticeable photoproduct selectivity.
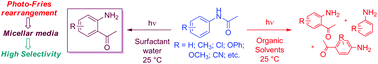

 Please wait while we load your content...
Please wait while we load your content...