Phototransformation of 3-alkoxychromenones: regioselective photocyclisation and dealkoxylation†
Abstract
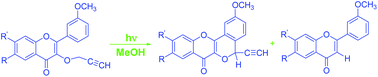
The phototransformation of some 2-(3-methoxyphenyl)-4H-chromen-4-ones bearing a propynyloxy moiety at the 3-position has been described. On photolysis with pyrex-filtered UV light from a Hg lamp (125 W), these chromenones produced a major amount of 5-ethynyl-2-methoxy-6-oxa-benzo[5,6-c]xanthen-7-ones consisting of an exotic tetracyclic scaffold. These photoproducts have been envisioned to be produced through regioselective ring closure at the 6′-position of the 2-(3′-methoxy)phenyl moiety of the initially formed 1,4-biradical via a γ-H abstraction mechanism. No product whatsoever was observed through ring closure at the 2′-position. This behaviour has been found to be in accordance with the directive influence observed in free radical aromatic substitutions. This regioselective photocyclisation is further supported by calculations made from 3D structures (MM2 program). In addition, during the irradiation of these substrates, 2-(3-methoxyphenyl)-4H-chromen-4-ones were also realised through dealkoxylation. The structures of the substrates and photoproduct(s) have been determined by their spectroscopic (IR, NMR, mass spectrometry) studies.

 Please wait while we load your content...
Please wait while we load your content...