Broadband ultrafast photoprotection by oxybenzone across the UVB and UVC spectral regions†
Abstract
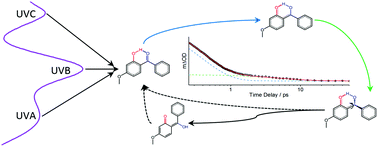
Recent studies have shed light on the energy dissipation mechanism of oxybenzone, a common ingredient in commercial sunscreens. After UVA photoexcitation, the dissipation mechanism may be understood in terms of an initial ultrafast excited state enol → keto tautomerisation, followed by nonadiabatic transfer to the ground electronic state and subsequent collisional relaxation to the starting enol tautomer. We expand on these studies using femtosecond transient electronic absorption spectroscopy to understand the non-radiative relaxation pathways of oxybenzone in cyclohexane and in methanol after UVB and UVC excitation. We find that the relaxation pathway may be understood in the same way as when exciting in the UVA region, concluding that oxybenzone displays proficient broadband non-radiative photoprotection, and thus photophysically justifying its inclusion in sunscreen mixtures.

 Please wait while we load your content...
Please wait while we load your content...