Enhanced Ga2O3-photocatalyzed and photochemical degradation of the Fipronil insecticide by UVC irradiation in mixed aqueous/organic media under an inert atmosphere
Abstract
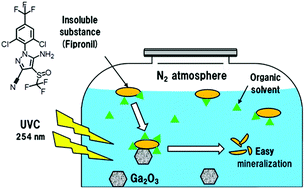
Agrochemicals such as the insecticide Fipronil that bear fluoro groups are generally fat-soluble and nearly insoluble in water, so that their photodegradation in a heterogeneous aqueous gallium oxide dispersion presents some challenges. This article examined the photodegradation of this insecticide by solubilizing it through the addition of organic solvents (EtOH, MeOH, THF, 1,4-dioxane and ethylene glycol) to an aqueous medium and then subjecting the insecticide to 254 nm UVC radiation under photocatalytically inert (Ga2O3/N2) and air-equilibrated (Ga2O3/O2) conditions, as well as photochemically in the absence of Ga2O3 but also under inert and air-equilibrated conditions. Defluorination, dechlorination, desulfonation and denitridation of Fipronil were examined in mixed aqueous/organic media (10, 25 and 50 vol% in organic solvent). After 3 h of UVC irradiation (50 vol% mixed media) defluorination with Ga2O3/N2 was ∼65% greater than in aqueous media, and ca. 80% greater than the direct photolysis of Fipronil under inert (N2) conditions; under air-equilibrated conditions both Ga2O3-photocatalyzed and photochemical defluorination were significantly lower than in aqueous media. Dechlorination of Fipronil was ∼160% (Ga2O3/N2) and 140% (photochemically, N2) greater than in aqueous media; under air-equilibrated conditions, both photocatalyzed and photochemical formation of Cl− ions in mixed media fell rather short relative to aqueous media. The photocatalyzed (Ga2O3/N2) and photochemical (N2) conversion of the sulfur group in Fipronil to SO42− ions was ca. 20% and 30% greater, respectively, in mixed media, while under air-equilibrated conditions photocatalyzed desulfonation was nearly twofold less than in the aqueous phase; direct photolysis showed little variations in mixed media. Denitridation of the nitrogens in Fipronil occurred mostly through the formation of ammonia (as NH4+) under all conditions with negligible quantities of NO3−; again mixed media offered enhanced denitridation, particularly under inert N2 conditions. Time-of-flight electrospray (TOF-MS/ESI−) mass spectrometry revealed a fairly large number of intermediates formed in the degradation of Fipronil, particularly under photocatalytic conditions. Only a couple of intermediates were identified in the photodegradation and the presence of Ga2O3 enhanced the complexity of an already cumbersome problem owing to the involvement of organic solvents.

 Please wait while we load your content...
Please wait while we load your content...