Regioselective synthesis of fullerene multiadducts via tether-directed 1,3-dipolar cycloaddition†
Abstract
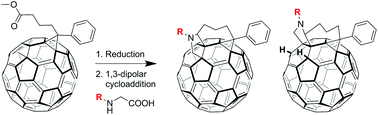
The regioselective synthesis of fullerene multiadducts was achieved from commercially available reagents in one pot over two steps. The configuration of the isolated regioisomers was determined using various NMR methods, UV-vis spectroscopy and electrochemical analysis with the structure of one isomer confirmed by single crystal X-ray analysis. Interesting variation in regioselectivity was observed when different amino acid reagents were used in the reactions. Theoretical calculations and additional experiments, such as deuterium exchange, led to a proposed mechanism for the regioselective product formation.


 Please wait while we load your content...
Please wait while we load your content...