Synthesis and electronic properties of π-extended flavins†
Abstract
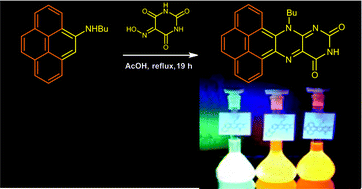
Flavin derivatives with an extended π-conjugation were synthesized in moderate to good yields from aryl bromides via a Buchwald–Hartwig palladium catalyzed amination protocol, followed by condensation of the corresponding aromatic amines with violuric acid. The electronic properties of the new compounds were investigated by absorption and emission spectroscopy, cyclic voltammetry, density functional theory (DFT) and time dependent density functional theory (TDDFT). The compounds absorb up to 550 nm and show strong luminescence. The photoluminescence quantum yields ϕPL measured in dichloromethane reach 80% and in PMMA (poly(methyl methacrylate)) 77%, respectively, at ambient temperature. The electrochemical redox behaviour of π-extended flavins follows the mechanism previously described for the parent flavin.

 Please wait while we load your content...
Please wait while we load your content...