Concise diastereoselective synthesis of calcaripeptide C via asymmetric transfer hydrogenation/Pd-induced chiral allenylzinc as a key reaction†
Abstract
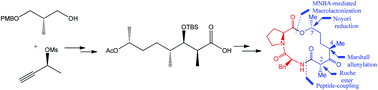
Synthesis of the natural product calcaripeptide C derived from the fungal metabolite mycelium KF525 of Calcarisporium sp. has been achieved. This complementary approach avoids the use of a stoichiometric amount of chiral auxiliary reagents as commonly used to generate enantioenriched advanced precursors. The enantioselective synthesis of calcaripeptide C is remarkable in that using catalytic reactions sets the two stereogenic centers efficiently with good levels of enantioselectivity. Further diversification of the calcaripeptide C structures is possible by employing a complementary catalytic enantioenriched Ru-catalyst.

 Please wait while we load your content...
Please wait while we load your content...