Uncatalysed diaryldiazo cyclopropanations on bicyclic lactams: access to annulated prolines†
Abstract
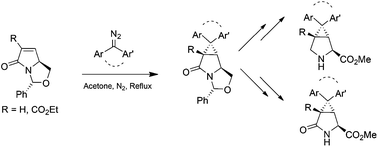
The uncatalysed cycloaddition of substituted diaryldiazo compounds onto bicyclic unsaturated lactams derived from pyroglutamic acid efficiently leads to highly functionalised azatricyclononanes. The products are readily elaborated to deprotected pyroglutamate derivatives, providing rapid access to conformationally constrained amino acids and their analogues. Preliminary assessment of antibacterial activity against one Gram positive and one Gram negative organism indicated high levels of efficacy in some cases.

 Please wait while we load your content...
Please wait while we load your content...