Hepatitis B virus peptide inhibitors: solution structures and interactions with the viral capsid†
Abstract
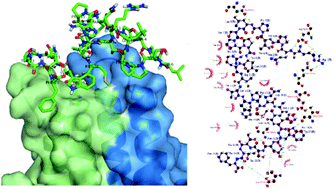
Hepatitis B virus (HBV) infection remains a health problem globally despite the availability of effective vaccines. In the assembly of the infectious virion, both the preS and S regions of the HBV large surface antigen (L-HBsAg) interact synergistically with the viral core antigen (HBcAg). Peptides preS and S based on the L-HBsAg were demonstrated as potential inhibitors to block the viral assembly. Therefore, the objectives of this study were to determine the solution structures of these peptides and study their interactions with HBcAg. The solution structures of these peptides were solved using 1H, 13C, and 15N NMR spectroscopy. Peptide preS has several structured regions of β-turns at Ser7-Pro8-Pro9, Arg11-Thr12-Thr13 and Ser22-Thr23-Thr24 sequences whereas peptide S has only one structured region observed at Ser3-Asn4-His5. Both peptides contain bend-like structures surrounding the turn structures. Docking studies revealed that both peptides interacted with the immunodominant region of HBcAg located at the tip of the viral capsid spikes. Saturation Transfer Difference (STD) NMR experiments identified several aromatic residues in peptides preS and S that interact with HBcAg. This study provides insights into the contact regions of L-HBsAg and HBcAg at atomic resolution which can be used to design antiviral agents that inhibit HBV morphogenesis.

 Please wait while we load your content...
Please wait while we load your content...