Synthesis of 3-aminoBODIPY dyes via copper-catalyzed vicarious nucleophilic substitution of 2-halogeno derivatives†
Abstract
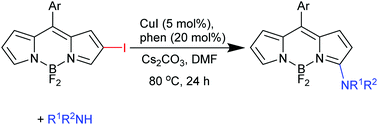
2-Halogeno BODIPYs undergo copper catalysed nucleophilic substitution with alkyl amines and anilines and an amide to give the corresponding 3-aminoBODIPY derivatives. The substrates are readily prepared by the regioselective 2-halogenation of the chemically robust, preformed BODIPYs thus providing an alternative to direct nucleophilic substitution of the corresponding 3-halogenoBODIPYs which requires regioselective 3-halogenation of the more sensitive dipyrromethane intermediate. 2-Halogenation expands the scope of vicarious substitution of BODIPYs to include weaker nitrogen nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...