Physicochemical studies on the copper(ii) binding by glycated collagen telopeptides†
Abstract
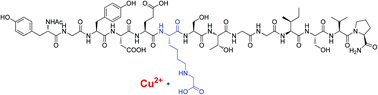
Emerging evidence indicates that levels of advanced glycation end-products (AGEs) correlate with age- and diabetes-related organ damage and may play a causative role in such damage. Increased chelation of Cu(II) ions appears to play an important role in this process, however, the precise relationship between formation of AGEs and accumulation of Cu(II) is yet to be determined. The interaction between AGEs and Cu(II) has been investigated using a collagenous peptide that has been site-specifically modified by a key AGE. Potentiometric titration showed that introduction of this AGE increased the capacity of the host-peptide to bind Cu(II). This result was confirmed by mass spectrometric characterisation of the AGE-modified peptide-Cu(II) system.

 Please wait while we load your content...
Please wait while we load your content...