Highly stable triple helix formation by homopyrimidine (l)-acyclic threoninol nucleic acids with single stranded DNA and RNA†
Abstract
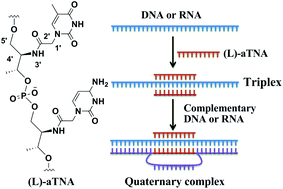
Acyclic (L)-threoninol nucleic acid (aTNA) containing thymine, cytosine and adenine nucleobases were synthesized and shown to form surprisingly stable triplexes with complementary single stranded homopurine DNA or RNA targets. The triplex structures consist of two (L)-aTNA strands and one DNA or RNA, and these triplexes are significantly stronger than the corresponding DNA or RNA duplexes as shown in competition experiments. As a unique property the (L)-aTNAs exclusively form triplex structures with DNA and RNA and no duplex structures are observed by gel electrophoresis. The results were compared to the known enantiomer (D)-aTNA, which forms much weaker triplexes depending upon temperature and time. It was demonstrated that (L)-aTNA triplexes are able to stop primer extension on a DNA template, showing the potential of (L)-aTNA for antisense applications.

 Please wait while we load your content...
Please wait while we load your content...