Synthesis of 1,4-amino alcohols by Grignard reagent addition to THF and N-tosyliminobenzyliodinane†
Abstract
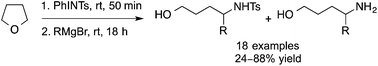
The synthesis of 1,4-amino alcohols from THF treated with N-tosyliminobenzyliodinane (PhINTs) followed by a Grignard reagent under mild reaction conditions at room temperature is described herein. Various Grignard reagents were shown to be compatible, furnishing the corresponding 4-substituted-N-1,4-tosylamino alcohols in good to excellent yields. A partial or full detosylation of the N-tosyl-1,4-amino alcohol was observed in instances involving a sterically bulky Grignard reagent, leading to the deprotected 1,4-amino alcohol product in moderate to good yields. The synthetic utility of this protocol was demonstrated by the synthesis of a 5-substituted-N-tosyl-1,5-amino alcohol from THP and the conversion of two examples to their corresponding γ-lactam and pyrrolidine adducts.

 Please wait while we load your content...
Please wait while we load your content...