A chemoselective α-aminoxylation of aryl ketones: a cross dehydrogenative coupling reaction catalysed by Bu4NI†
Abstract
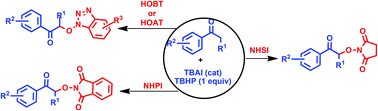
Tetrabutyl ammonium iodide (TBAI) catalyzed α-aminoxylation of ketones using aq. TBHP as an oxidant has been accomplished. We have shown that the CDC (cross dehydrogenative coupling) reactions of ketones with N-hydroxyimidates such as N-hydroxysuccinimide (NHSI), N-hydroxyphthalimide (NHPI), N-hydroxybenzotriazole (HOBt) and 1-hydroxy-7-azabenzotriazole (HOAt) lead to the corresponding oxygenated products in good to moderate yields. The application of this method has been demonstrated by transforming a few coupled products into synthetically useful intermediates and products.

 Please wait while we load your content...
Please wait while we load your content...