Complementary isonitrile-based multicomponent reactions for the synthesis of diversified cytotoxic hemiasterlin analogues†
Abstract
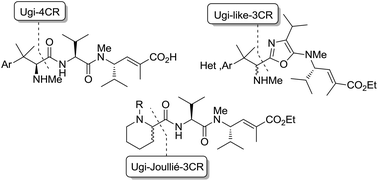
A small family of structural analogues of the antimitotic tripeptides, hemiasterlins, have been designed and synthesized as potential inhibitors of tubulin polymerization. The effectiveness of a multicomponent approach was fully demonstrated by applying complementary versions of the isocyanide-based Ugi reaction. Compounds strictly related to the lead natural products, as well as more extensively modified analogues, have been synthesized in a concise and convergent manner. In some cases, biological evaluation provided evidence for strong cytotoxic activity (six human tumor cell lines) and for potent inhibition of tubulin polymerization.

 Please wait while we load your content...
Please wait while we load your content...