Total synthesis and absolute stereochemistry of the proteasome inhibitors cystargolides A and B†
Abstract
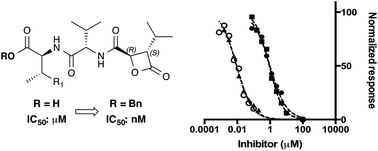
The absolute stereochemistry of the cystargolides was determined by total synthesis. Evaluation of synthetic cystargolides and derivatives showed that the natural (2S,3R) stereochemistry is essential for activity. Moreover, benzyl esters (−)-10 and (−)-15 were found to be about 100 times more potent, and to selectively kill MCF-7 cancerous cells.

 Please wait while we load your content...
Please wait while we load your content...