The development of a short route to the API ropinirole hydrochloride†
Abstract
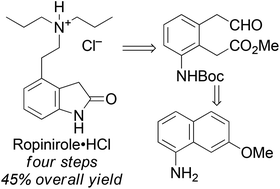
A four-step, three-stage synthesis of the API ropinirole hydrochloride has been developed from a commercially available naphthalene derivative. The new route has half the step-count and twice the overall yield of the current manufacturing process. Key features of the synthesis are a regioselective Birch reduction and an ozonolysis with concomitant ring closure to induce the required ring contraction.

 Please wait while we load your content...
Please wait while we load your content...