Synthesis and antimicrobial activity of binaphthyl-based, functionalized oxazole and thiazole peptidomimetics†
Abstract
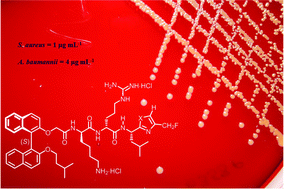
Thirty two new binaphthyl-based, functionalized oxazole and thiazole peptidomimetics and over thirty five novel leucine-containing intermediate oxazoles and thiazoles were prepared in this study. This includes the first examples of the direct C-5 arylation of an amino acid dipeptide-derived oxazole. Moderate to excellent antibacterial activity was observed for all new compounds across Gram positive isolates with MICs ranging from 1–16 μg mL−1. Results for Gram negative E. coli and A. baumannii were more variable, but MICs as low as 4 μg mL−1 were returned for two examples. Significantly, the in vitro results with a fluoromethyl-oxazole derivative collectively represent the best obtained to date for a member of our binaphthyl peptide antimicrobials.

 Please wait while we load your content...
Please wait while we load your content...