Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides†
Abstract
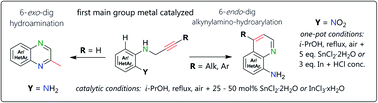
Pyrazino compounds such as quinoxalines are 1,4-diazines with widespread occurrence in nature. Quinolin-8-amines are isomerically related and valuable scaffolds in organic synthesis. Herein, we present intramolecular main group metal Lewis acid catalyzed formal hydroamination as well as hydroarylation methodology using mono-propargylated aromatic ortho-diamines. The annulations can be conducted utilizing equal aerobic conditions with either stannic chloride or indium(III) chloride and represent primary examples for main group metal catalyzed 6-exo-dig and 6-endo-dig, respectively, cyclizations in such settings. Both types of reactions can also be utilized in a one-pot manner starting from ortho-nitro N-propargyl anilines using stoichiometric amounts SnCl2·2H2O or In powder. Mechanistic considerations are presented regarding the substituent-depending regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...