Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones†
Abstract
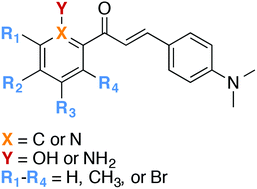
As the number of individuals affected with Alzheimer's disease (AD) increases and the availability of drugs for AD treatment remains limited, the need to develop effective therapeutics for AD becomes more and more pressing. Strategies currently pursued include inhibiting acetylcholinesterase (AChE) and targeting amyloid-β (Aβ) peptides and metal-Aβ complexes. This work presents the design, synthesis, and biochemical evaluation of a series of chalcones, and assesses the relationship between their structures and their ability to bind metal ions and/or Aβ species, and inhibit AChE/BChE activity. Several chalcones were found to exhibit potent disaggregation of pre-formed N-biotinyl Aβ1-42 (bioAβ42) aggregates in vitro in the absence and presence of Cu2+/Zn2+, while others were effective at inhibiting the action of AChE.

 Please wait while we load your content...
Please wait while we load your content...