Dicyanovinyl-substituted J147 analogue inhibits oligomerization and fibrillation of β-amyloid peptides and protects neuronal cells from β-amyloid-induced cytotoxicity†
Abstract
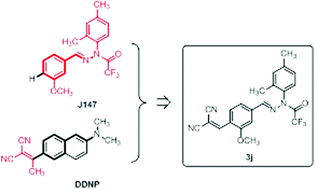
A series of novel J147 derivatives were synthesized, and their inhibitory activities against β-amyloid (Aβ) aggregation and toxicity were evaluated by using the oligomer-specific antibody assay, the thioflavin-T fluorescence assay, and a cell viability assay in the transformed SH-SY5Y cell culture. Among the synthesized J147 derivatives, 3j with a 2,2-dicyanovinyl substituent showed the most potent inhibitory activity against Aβ42 oligomerization (IC50 = 17.3 μM) and Aβ42 fibrillization (IC50 = 10.5 μM), and disassembled the preformed Aβ42 fibrils with an EC50 of 10.2 μM. Finally, we confirmed that 3j is also effective at preventing neurotoxicity induced by Aβ42-oligomers as well as Aβ42-fibrils.

 Please wait while we load your content...
Please wait while we load your content...