One-pot synthesis of GABA amides via the nucleophilic addition of amines to 3,3-disubstituted cyclopropenes†
Abstract
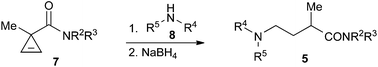
A one-pot synthesis of various GABA amides has been demostrated, employing the nucleophilic addition of primary and secondary amines across the double bond of cyclopropene-3-carboxamides, followed by ring-opening of the resulting donor–acceptor cyclopropanes and subsequent in situ reduction of enamine (imine) intermediates.

 Please wait while we load your content...
Please wait while we load your content...