d-Glucosamine as a novel chiral auxiliary for the stereoselective synthesis of P-stereogenic phosphine oxides†
Abstract
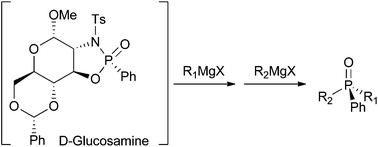
D-Glucosamine was successfully employed as a chiral auxiliary for the enantioselective synthesis of phosphine oxides. The influence of the anomeric position was also investigated and revealed the excellent ability of the α-anomer to perform this transformation in a highly selective fashion. The methodology employed consisted of three steps: diastereoselective formation of the oxazaphospholidine followed by subsequent selective cleavage of P–N and P–O bonds by reaction with two Grignard reagents. P-epimers oxazaphospholidines were prepared switching from a P(V) to a P(III) precursor, thus allowing for the synthesis of enantiomeric phosphine oxides. In addition, the chiral auxiliary could be recovered and efficiently recycled.

 Please wait while we load your content...
Please wait while we load your content...