Aziridine electrophiles in the functionalisation of peptide chains with amine nucleophiles†
Abstract
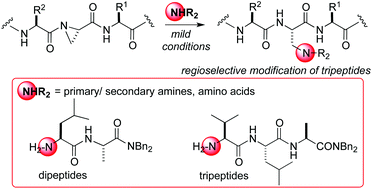
We describe herein the synthesis of aziridine-containing amino acids embedded within tripeptide structures. A range of amine nucleophiles have been shown to open the aziridine amino acid regioselectively at the β-position under mild conditions and without the requirement for a catalyst, forming new adducts in the process. Amino acid N-termini (or an N-containing sidechain) also served as effective nucleophiles for such aziridines and this concept could be extended to encompass a di- or tripeptide nitrogen as a nucleophile, thus providing new methodology for linking together peptide strands using an amine linker.

 Please wait while we load your content...
Please wait while we load your content...