Palladium-catalyzed ortho-acyloxylation of N-nitrosoanilines via direct sp2 C–H bond activation†
Abstract
The palladium-catalyzed N-nitroso-directed ortho-acyloxylation of N-nitrosoanilines has been demonstrated via sp2 C–H activation with a stoichiometric amount of PhI(OAc)2 as the oxidant and Ac2O/AcOH (1 : 1) or C2H5CO2H as the reaction medium. This protocol can be applied to various N-nitrosoanilines with both electron-donating and electron-withdrawing groups. In addition, the products can be further transformed to 2-(methylamino)phenols expediently by a simple reduction method.
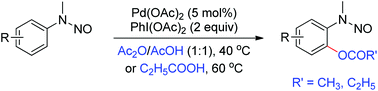

 Please wait while we load your content...
Please wait while we load your content...