A mild and facile synthesis of polyfunctionalized pyridines: merging three-component cyclization and aerobic oxidation by amine/metal catalysts†
Abstract
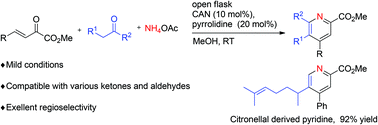
A mild and facile synthesis of polyfunctionalized pyridines from NH4OAc, β,γ-unsaturated α-ketoesters, and ketones/aldehydes has been reported through tandem three-component cyclization and aerobic oxidation using the combination of amine and metal catalysts. The synthetic value of the developed methodology was demonstrated by a gram-scale reaction and a wide range of substrates including naturally occurring citronellal.

 Please wait while we load your content...
Please wait while we load your content...