Synthesis and evaluation of cationic norbornanes as peptidomimetic antibacterial agents†
Abstract
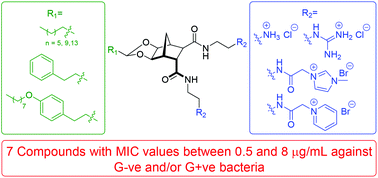
A series of structurally amphiphilic biscationic norbornanes have been synthesised as rigidified, low molecular weight peptidomimetics of cationic antimicrobial peptides. A variety of charged hydrophilic functionalities were attached to the norbornane scaffold including aminium, guanidinium, imidazolium and pyridinium moieties. Additionally, a range of hydrophobic groups of differing sizes were incorporated through an acetal linkage. The compounds were evaluated for antibacterial activity against both Gram-negative and Gram-positive bacteria. Activity was observed across the series; the most potent of which exhibited an MIC's ≤ 1 μg mL−1 against Streptococcus pneumoniae, Enterococcus faecalis and several strains of Staphylococcus aureus, including multi-resistant methicillin resistant (mMRSA), glycopeptide-intermediate (GISA) and vancomycin-intermediate (VISA) S. aureus.

 Please wait while we load your content...
Please wait while we load your content...