RNA nucleosides as chiral sensing agents in NMR spectroscopy†
Abstract
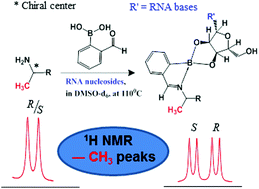
The study reports chiral sensing properties of RNA nucleosides. Adenosine, guanosine, uridine and cytidine are used as chiral derivatizing agents to differentiate chiral 1°-amines. A three component protocol has been adopted for complexation of nucleosides and amines. The chiral differentiating ability of nucleosides is examined for different amines based on the 1H NMR chemical shift differences of diastereomers (ΔδR,S). Enantiomeric differentiation has been observed at multiple chemically distinct proton sites. Adenosine and guanosine exhibit large chiral differentiation (ΔδR,S) due to the presence of a purine ring. The diastereomeric excess (de) measured by using adenosine is in good agreement with the gravimetric values.

 Please wait while we load your content...
Please wait while we load your content...