Synthetic studies toward the brasilinolides: controlled assembly of a protected C1–C38 polyol based on fragment union by complex aldol reactions†
Abstract
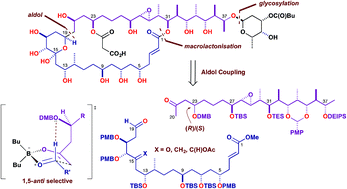
The brasilinolides are an architecturally complex family of 32-membered macrolides, characterised by potent immunosuppressant and antifungal properties, which represent challenging synthetic targets. By adopting a highly convergent strategy, a range of asymmetric aldol/reduction sequences and catalytic protocols were employed to assemble a series of increasingly elaborate fragments. The controlled preparation of suitable C1–C19 and C20–C38 acyclic fragments 5 and 6, containing seven and 12 stereocentres respectively, was first achieved. An adventurous C19–C20 fragment union was then explored to construct the entire carbon chain of the brasilinolides. This pivotal coupling step could be performed in a complex boron-mediated aldol reaction to install the required C19 hydroxyl stereocentre when alternative Mukaiyama-type aldol protocols proved unrewarding. A protected C1–C38 polyol 93 was subsequently prepared, setting the stage for future late-stage diversification toward the various brasilinolide congeners. Throughout this work, asymmetric boron-mediated aldol reactions of chiral ketones with aldehydes proved effective both for controlled fragment assembly and coupling with predictable stereoinduction from the enolate component.

 Please wait while we load your content...
Please wait while we load your content...