Facile synthesis and stereo-resolution of chiral 1,2,3-triazoles†
Abstract
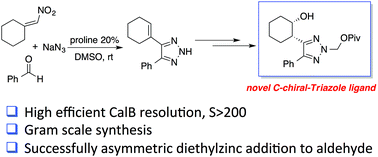
We describe herein the first facile synthesis of chiral triazoles through side chain functionalization. Readily available C-vinyl triazoles were used as starting materials, followed by sequential epoxidation, rearrangement and reduction to give the corresponding hydroxyl-triazoles. The CalB lipase catalyzed kinetic resolution gave enantiomerically pure (>99% ee) chiral triazoles in excellent yield. These new chiral TAs were also successfully applied as ligands in asymmetric addition of diethylzinc to aldehydes.

 Please wait while we load your content...
Please wait while we load your content...