Design and synthesis of analogues of natural products†
Abstract
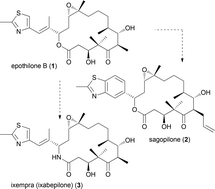
In this article strategies for the design and synthesis of natural product analogues are summarized and illustrated with some selected examples. Proven strategies include diverted total synthesis (DTS), function-oriented synthesis (FOS), biology-oriented synthesis (BIOS), complexity to diversity (CtD), hybrid molecules, and biosynthesis inspired synthesis. The latter includes mutasynthesis, the synthesis of natural products encoded by silent genes, and propionate scanning. Most of the examples from our group fall in the quite general concept of DTS. Thus, in case an efficient strategy to a natural product is at hand, modifications are possible at almost any stage of a synthesis. However, even for compounds of moderate complexity, organic synthesis remains a bottle neck. Unless some method for predicting the biological activity of a designed molecule becomes available, the design and synthesis of natural product analogues will remain what it is now, namely it will largely rely on trial and error.

 Please wait while we load your content...
Please wait while we load your content...