Alkynylation of heterocyclic compounds using hypervalent iodine reagent†
Abstract
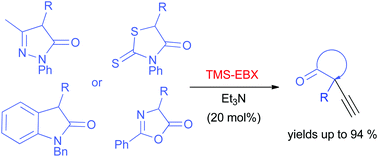
The alkynylation of various nitrogen- and/or sulphur-containing heterocyclic compounds using hypervalent iodine TMS-EBX by utilization of tertiary amines under mild conditions is described. The developed metal-free methodology furnishes the corresponding alkynylated heterocycles bearing quaternary carbon in high yields.

 Please wait while we load your content...
Please wait while we load your content...