Molecular motion of donor–acceptor catenanes in water†
Abstract
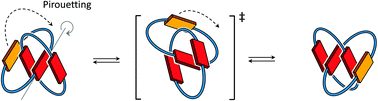
In this article, we use 1H NMR spectroscopy to study the spontaneous molecular motion of donor–acceptor [2]catenanes in water. Our data supports the hypothesis that conformational motion dominantly occurs through a pirouetting mechanism, which involves less exposure of hydrophobic surfaces than in a rotation mechanism. Motion is controlled by the size of the catenane rings and the arrangement of the electron-deficient and electron-rich aromatic units.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...