Exploring the scope of the isothiourea-mediated synthesis of dihydropyridinones†
Abstract
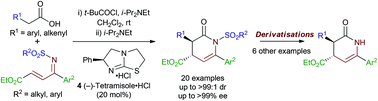
The exploration and expansion of the scope of the isothiourea-mediated synthesis of dihydropyridinones is presented. The use of ketimines derived from α,β-unsaturated γ-ketoesters as the Michael acceptor in a Michael addition/lactamisation cascade gives access to a range of dihydropyridinones with high enantioselectivity. The nature of the N-sulfonyl group present on the ketimine is extensively investigated, with further studies into derivatisation of the dihydropyridinone core also reported.
 Please wait while we load your content...
Please wait while we load your content...