Homocoupling versus reduction of radicals: an experimental and theoretical study of Ti(iii)-mediated deoxygenation of activated alcohols†
Abstract
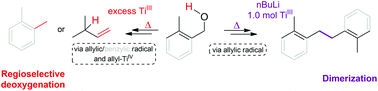
A detailed experimental and theoretical study corroborates that the reductive deoxygenation of activated (allylic or benzylic) alcohols with excess Ti(III) proceeds via an allyl(benzyl)-radical and allyl(benzyl)-Ti, which is protonated, regioselectively in the case of allylic derivatives. The H atom of the newly formed C–H bond in the product originates from the –OH group of the starting material. The deoxygenation of lithium alkoxides or alcohols by using 1.0 mol of Ti(III) leads to the corresponding dimerization products in good yields. An excellent agreement with the experimental data was obtained by using a reaction kinetics simulator to discriminate between competing reactions.

 Please wait while we load your content...
Please wait while we load your content...