Multicomponent one-pot synthesis of highly-functionalized pyrrole-3-carbonitriles in aqueous medium and their computational study†
Abstract
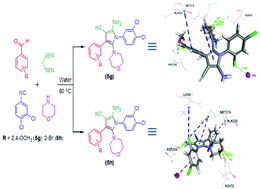
A one-pot green protocol involving four-components in aqueous medium is developed to synthesize highly-functionalized pyrroles in good yields. Two of the newly synthesized compounds were subjected to in silico analysis on the Pharm Mapper web-server and the human mitogen-activated protein kinase1 (MEK1) enzyme was identified as a potential target protein for these compounds. For target validation, MEK-1 inhibition was performed with two representative compounds (5g and 5h) using docking simulations. These functionalized pyrroles were also tested for their respective IC50 values on human cancer cell lines to evaluate their biocompatibility. Several of these functionalized pyrrole molecules were found to possess higher growth inhibition activity than standard doxorubicin and cisplatin.

 Please wait while we load your content...
Please wait while we load your content...