E- or Z-Selective synthesis of 4-fluorovinyl-1,2,3-triazoles with fluorinated second-generation Julia–Kocienski reagents†
Abstract
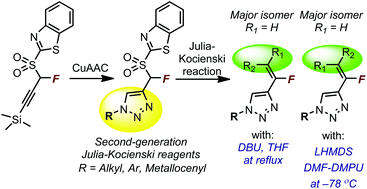
A highly modular approach to N-substituted 4-(1-fluorovinyl)triazoles is described. In situ desilylation and Cu-catalyzed ligation reaction of TMS-protected α-fluoropropargyl benzothiazole sulfone with aryl, alkyl, and metallocenyl azides furnished second-generation Julia–Kocienski reagents in good to excellent yields. Condensation reactions of these reagents with aldehydes can be tuned to yield E or Z-alkenes selectively. Under mild conditions with DBU as the base, reactions of aldehydes furnished E-alkenes as the major isomer. On the other hand, in condensation reactions with LHMDS as the base and in appropriate solvents, both aldehydes and ketones reacted to yield fluoroalkenes with Z-selectivity. Stereochemical assignment of E/Z olefins obtained in the reaction of a ketone with two Julia reagents was performed via X-ray crystallographic analysis and comparisons of NMR data. The method allows efficient and ready diversification of the N1-substituent and substituents at the double bond.

 Please wait while we load your content...
Please wait while we load your content...