Determination of the absolute configuration of phosphinic analogues of glutamate†
Abstract
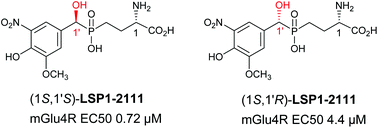
A series of phosphinic glutamate derivatives (e.g.LSP1-2111) have been proven to be potent agonists of metabotropic glutamate (mGlu) receptors and shown promising in vivo activity. However, so far all were synthesized and tested as a mixture of two diastereomers whose absolute and relative configurations are not known. In this study, the stereomers were separated on a Crownpack CR(+) column and their absolute configuration was assessed by means of a diastereoselective synthesis. Both separated L-stereomers activated the mGlu4 receptor with EC50's of 0.72 and 4.4 μM for (1S,1′S)-and (1S,1′R)-LSP1-2111, respectively.

 Please wait while we load your content...
Please wait while we load your content...