Synthesis of ordered mesoporous crystalline CuS and Ag2S materials via cation exchange reaction†
Abstract
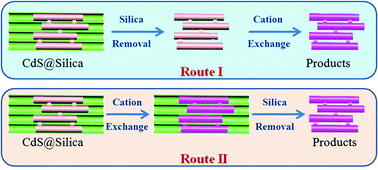
Cation exchange reaction is a strong tool for the synthesis of new ionic nanomaterials. Most of them are isolated nanoparticles with simple geometric features, such as nanodots, nanorods and nanospheres. In this work, we demonstrated that ordered mesoporous CdS with a complex cubic Ia3d gyroidal 3D bicontinuous porous structure and large particle size can be successfully converted to crystalline CuS and Ag2S materials via cation exchange reaction without destroying the well-defined nanostructure. The change in crystal structure is an important factor for a successful conversion when the reaction is carried out without the presence of a silica template. In addition, the cation exchange reaction is sufficient for a complete compositional conversion, even when the mesostructured CdS precursor is embedded inside a mesoporous silica matrix. Our results indicate that cation exchange reaction may be applied to highly complex nanostructures with extremely large particle sizes.

 Please wait while we load your content...
Please wait while we load your content...