Self-assembly of metal atoms (Na, K, Ca) on graphene†
Abstract
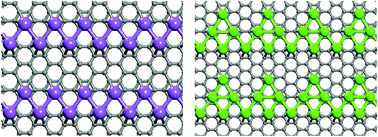
A thorough search of the distribution pattern of Na, K, and Ca atoms on graphene surface, carried out using a synergistic combination of density functional theory and particle swarm optimization algorithm, yielded some unusual results. The equilibrium distribution is concentration and metal dependent; the metal atoms distribute uniformly when their coverage ratio M : C (M = Na, K, Ca) is 1 : 6, but Na and Ca atoms self-assemble to form parallel quasi-one-dimensional chains when their coverage is reduced to 1 : 8. At the higher concentration (M : C = 1 : 6), electron–phonon coupling calculations further show that the NaC6 is a superconductor with critical temperature of 5.8 K, which is the highest value among all the stable alkali or alkaline-earth metal decorated monolayer graphene systems studied to-date. At the lower concentration (M : C = 1 : 8) and depending on metal species, well-aligned atomic metal chains interact with graphene with varying intensity, making it possible to achieve either rigid or non-rigid band doping in graphene.

 Please wait while we load your content...
Please wait while we load your content...