Conformation–activity relationships of polyketide natural products
Abstract
Covering: up to the end of 2014
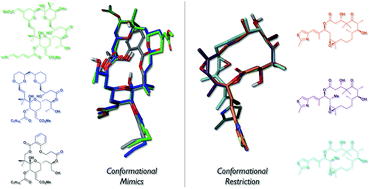
Polyketides represent an important class of secondary metabolites that interact with biological targets connected to a variety of disease-associated pathways. Remarkably, nature's assembly lines, polyketide synthases, manufacture these privileged structures through a combinatorial mixture of just a few structural units. This review highlights the role of these structural elements in shaping a polyketide's conformational preferences, the use of computer-based molecular modeling and solution NMR studies in the identification of low-energy conformers, and the importance of conformational analogues in probing the bound conformation. In particular, this review covers several examples wherein conformational analysis complements classic structure–activity relationships in the design of biologically active natural product analogues.

 Please wait while we load your content...
Please wait while we load your content...